Abstract
Aims/hypothesis
Adaptation to energy restriction is associated with changes in gene expression in adipose tissue. However, it is unknown to what extent these changes are dependent on the energy restriction as such or on the macronutrient composition of the diet.
Methods
We determined the levels of transcripts for 38 genes that are expressed in adipose tissue and encode transcription factors, enzymes, transporters and receptors known to play critical roles in the regulation of adipogenesis, mitochondrial respiration, and lipid and carbohydrate metabolism. Two groups of 25 obese subjects following 10-week hypocaloric diet programmes with either 20–25 or 40–45% of total energy derived from fat were investigated. Levels of mRNA were measured by performing real-time RT-PCR on subcutaneous fat samples obtained from the subjects before and after the diets.
Results
The two groups of subjects lost 7 kg over the duration of the diets. Ten genes were regulated by energy restriction; however, none of the genes showed a significantly different response to the diets. Levels of peroxisome proliferator-activated receptor γ co-activator 1α mRNA were increased, while the expression of the genes encoding leptin, osteonectin, phosphodiesterase 3B, hormone-sensitive lipase, receptor A for natriuretic peptide, fatty acid translocase, lipoprotein lipase, uncoupling protein 2 and peroxisome proliferator-activated receptor γ was decreased. Clustering analysis revealed new potential coregulation of genes. For example, the expression of the genes encoding the adiponectin receptors may be regulated by liver X receptor α.
Conclusions/interpretation
In accordance with the comparable loss of fat mass produced by the two diets, this study shows that energy restriction and/or weight loss rather than the ratio of fat: carbohydrate in a low-energy diet is of importance in modifying the expression of genes in the human adipose tissue.
Similar content being viewed by others
Introduction
Obesity is a major risk factor for type 2 diabetes and weight reduction is an important component of the therapy of the diabetic obese patient. Hypocaloric diets are widely used to promote the negative energy balance necessary for to reduce fat mass. There has been considerable interest in dietary composition and weight loss [1]; however, it remains to be determined which ratio of lipid : carbohydrate in a low-calorie diet most effectively facilitates weight loss or improves metabolic profiles. A low-fat diet is generally considered to be better than a high-fat diet because the patient will benefit from reduced cardiovascular risk as a result of weight loss and a restricted fat intake. On the other hand, some subjects are more compliant when placed on high-fat hypocaloric diets [2]. There have been few studies on the effect of altering the fat and carbohydrate content of hypocaloric diets [3–7]. The small number of subjects included in these studies has precluded the detection of clinically relevant differences in weight loss and body composition. We recently performed the Nutrient–Gene Interactions in Human Obesity—Implications for Dietary Guidelines (NUGENOB) trial on 771 obese subjects. This was a large randomised intervention trial that was designed to study the effects of low-fat, high-carbohydrate and high-fat, low-carbohydrate hypocaloric diets (unpublished results). The results indicated that the high-fat diet was as effective as the low-fat diet in producing weight loss. Changes in fat mass, fat-free mass, and waist and hip circumferences were not significantly different between the two diet groups.
Little is known about the ways in which macronutrients and energy restriction affect the regulation of adipose tissue gene expression. Changes in the levels of a few different mRNAs have been determined during 4-week very-low-calorie diets [8–14] and 10-week low-calorie diets [15]. To date, the determinants of gene expression in adipose tissue during hypocaloric diets with different nutrient compositions have not been investigated in vivo. Much progress has been made towards the elucidation of the molecular mechanisms responsible for the effects of nutrients on gene expression. Fatty acids have been found to utilise several pathways [16, 17], and a glucose-responsive transcription factor has been characterised [18, 19]. In addition, recent studies have investigated pathways that mediate the effect of calorie restriction in mammals [20, 21]. However, the extent to which changes in adipose tissue gene expression are dependent on the calorie restriction as such or on the composition of the diet remains unknown.
The aim of the present study was to investigate the importance of nutrient composition and energy restriction on the regulation of adipose tissue gene expression among obese subjects enrolled in the NUGENOB programme. The genes analysed were selected from different functional categories of adipose tissue biology (Table 1). We studied the expression of genes encoding transcription factors, enzymes, transporters and receptors that are known to play critical roles in the regulation of adipogenesis, mitochondrial respiration, and lipid and carbohydrate metabolism. Transcripts encoding peptides secreted by adipose tissue that act on other organs were also investigated.
Subjects and methods
Subjects and design
Subjects were participants in the European multicentre NUGENOB study (www.nugenob.com), which was supported by the European Community. Informed consent was obtained from all subjects. Clinical investigations were approved by the ethical committees of each participating centre and were performed according to the Declaration of Helsinki. Subjects were randomly assigned to one of two similarly energy-restricted diets: a high-fat, low-carbohydrate diet or a low-fat, high-carbohydrate diet. The present study included 50 women chosen at random from the 771 obese patients who participated in the initial study. Subjects were equally distributed between the diets and the eight European clinical centres. The patients were 21–49 years old and had a BMI >30 kg/m2 (mean BMI 36.2±0.7 kg/m2). During the dietary intervention the subjects either visited or had telephone contact with the dietician every week. The dietician then assessed the compliance of the subjects and checked the content of their diets from their food diaries. The subjects also completed a 3-day weighed food record for two weekdays and one weekend day before the start of the dietary intervention and at the end of the 10-week diet. This was done to assess the habitual diets of the subjects and to estimate their compliance, respectively. Subjects completed 1-day weighed food records during the second, fifth and seventh weeks of the intervention. The food records were analysed using a food nutrient database. The two diets decreased energy intake to the same extent (from 2271±627 to 1567±313 kcal/day on the high-fat diet, and from 2301±554 to 1617±554 kcal/day on the low-fat diet). In the low-fat diet, 24.4±3.2% of the total energy intake was derived from lipids (saturated, monounsaturated and polyunsaturated fatty acids constituted 9.2±2.3, 9.8±2.3 and 5.4±1.4% of the total energy intake, respectively), 34.2±8.2% was from polysaccharides, and 24.6±6.9% was from simple sugars. In the high-fat diet, 41.6±3.6% of the total energy intake was supplied by lipids (saturated, monounsaturated and polyunsaturated fatty acids constituted 15.3±3.6, 18.4±3.9 and 7.9±3.0% of the total energy intake, respectively), 25.1±4.7% was from polysaccharides, and 15.0±8.4% was from simple sugars. The subjects were weighed when they visited the centres on every second week of the study.
The total energy expenditure for each subject was estimated as follows. The resting metabolic rate, measured using a ventilated hood system, was expressed in kcal/day and was multiplied by 1.3. From this value, 600 kcal were subtracted to obtain the figure for a moderately hypocaloric diet. In the low-fat diet, 20–25% of total energy was provided by fat; the corresponding figure for the high-fat diet was 40–45%. Both diets derived 15% of total energy from protein and the remainder (60–65% and 40–45% for the low-fat and high-fat diets, respectively) from carbohydrates. Fat mass and fat-free mass were assessed using multifrequency bioimpedance (QuadScan 4000; Bodystat, Douglas, Isle of Man, British Isles). Plasma leptin and NEFA levels were determined using the human leptin RIA kit (Linco research, St. Charles, MO, USA) and the NEFA-C kit (Wako Chemicals, Neuss, Germany), respectively.
Quantitation of mRNA
Before and after the dietary intervention, biopsies of subcutaneous abdominal adipose tissue (∼1 g) were performed under local anaesthesia following an overnight fast. The samples were washed, soaked in RNAlater preservative solution (Qiagen, Courtaboeuf, France) and stored at −80°C until analysis. Total RNA was extracted from subcutaneous adipose tissue biopsies using the RNeasy total RNA Mini kit (Qiagen). The integrity of total RNA was systematically checked by electrophoresis through an agarose gel. After DNase I treatment, 1 μg of total RNA was reverse transcribed using random hexamers and poly(dT) as primers and Superscript II reverse transcriptase (Invitrogen, Cergy Pontoise, France). Quantitative real-time PCR was performed using a GeneAmp 7000 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) or a LightCycler (Roche Diagnostics, Meylan, France). For each primer pair, a standard curve was obtained using serial dilutions of human adipose tissue cDNA prior to mRNA quantitation. To control for contamination by genomic DNA, quantitative PCR was performed on reverse transcription reactions without reverse transcriptase. The negative reverse transcription reactions had cycle threshold (Ct) values >40, and the difference between the negative and positive reactions was >10 Ct. All reactions were performed in duplicate. When the difference between the duplicates was above 0.5 Ct, quantitative PCR was repeated. We used 18S rRNA as a control to normalise gene expression using the Ribosomal RNA Control TaqMan Assay kit (Applied Biosystems). We determined the mRNA levels of 38 genes (Table 1). A list of primers and PCR conditions are available upon request.
Statistical analysis
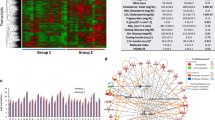
The overall effect of the energy restriction was tested using the paired Student’s t-test, and gene expression data were logarithmically transformed prior to analysis. General linear model univariate analysis was used to study the differential effect of the diet, with adjustment for baseline mRNA level (week 0) to increase precision and statistical power [22]. The model included adjustment for clinical centres. To control for changes in other parameters that could hide the differential effect of the diets (e.g., changes in BMI and fat mass), these variables were entered independently into the model. A p value less than or equal to 0.05 was considered statistically significant. All analyses were performed using SPSS for Windows, Version 11.5 (SPSS, Chicago, IL, USA). Hierarchical cluster analysis was performed using Euclidean distances as an estimate of similarity between two genes and Ward’s method to join groups of genes [23, 24]. Cluster analysis is different to the Student’s t-test in that it is used to identify similar variations as opposed to significant changes. The dendrogram obtained using the Ward algorithm (Fig. 1) was drawn from the percentage changes in mRNA levels (mRNA level at week 10 minus mRNA level at week 0 divided by mRNA level at week 0).
Dendrogram of similarities in patterns of gene expression in human adipose tissue during a hypocaloric diet. The relative changes in levels of the different mRNAs between baseline and week 10 of the diets were calculated and a hierarchical cluster analysis was performed using Euclidean distances and Ward’s method. The four main nodes are lettered on the graph. Please refer to Table 1 for the full names of the proteins encoded by the genes studied
Results
Clinical data
The anthropometric and clinical characteristics of the subjects are shown in Table 2. The diets reduced body weight by 6.75±0.45 kg and significantly decreased plasma leptin by 29%. The changes observed in weight and fat mass after 10 weeks were similar in the two groups. Both the high-fat and low-fat groups achieved a significant reduction in weight (6.7 and 6.8%, respectively) and fat mass (13.3 and 14.9%, respectively). Analysis of covariance using diet as a fixed factor did not show a significant effect of the type of diet on the anthropometric or metabolic parameters analysed except for plasma NEFA (p=0.051). Circulating NEFA levels, LDL cholesterol and HDL cholesterol decreased during low-fat diet. The quantitative insulin sensitivity check index (QUICKI), an indicator of insulin sensitivity, was significantly increased in both groups.
Gene expression data
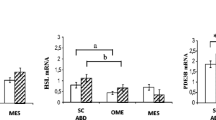
We investigated changes in the levels of expression of 38 genes with important roles in adipocyte biology (Table 1). A general linear model univariate analysis was performed to investigate the differential effect of the two types of diets on gene expression. The model was adjusted for baseline levels of mRNA to control for inter-individual variations in expression. To control for changes in important baseline determinants of gene expression that are modified during hypocaloric diets, BMI, body fat, NEFA and leptin levels were entered separately into the model. None of the genes were identified as being differentially regulated by the diets (p>0.1 for all), and the addition of the anthropometric and plasma variables did not modify the results (data not shown). In a second step we studied the overall effect of the 10-week moderate energy restriction on gene expression in human adipose tissue. Table 3 shows that the mRNA levels of ten genes were significantly changed. Levels of osteonectin, phosphodiesterase 3B, receptor A for natriuretic peptide (ANP-Ra), fatty acid translocase/CD36, uncoupling protein 2, lipoprotein lipase, leptin, hormone-sensitive lipase and peroxisome proliferator-activated receptor γ2 (PPARγ2) mRNAs were lower after 10 weeks of food restriction. Conversely, levels of the transcript encoding PPARγ co-activator 1α (PGC-1α) were increased. The changes in levels of mRNAs encoding PGC-1α, leptin, lipoprotein lipase and osteonectin were highly significant (p<0.001). These findings were not modified by the inclusion of the baseline determinants of gene expression in the analysis.
We used hierarchical clustering to ascertain which of the genes exhibited similar patterns of expression. The classification algorithm was used to group the genes according to their similarity in variations in mRNA levels. This provided cluster dendrograms that organised our observed data into meaningful structures. Of the many methods available for grouping data into clusters we chose the Ward method which unifies groups in such a way that the intra-group variability is minimised [23, 24]. This ensures that the resulting groups are as homogenous as possible and that the between-group differences are marked. Figure 1 shows the hierarchical clustering of the 38 genes according to changes in their levels of expression during the hypocaloric diets. Four master groups can be distinguished. Each group contains genes that show similar patterns of expression. Some nodes contained transcription factors together with other genes suggesting that the transcription factor could control the metabolic genes. One of the nodes contained the eight genes that were downregulated during calorie restriction.
Discussion
Although energy-restricted diets are often prescribed to obese non-diabetic subjects and obese type 2 diabetic subjects, little information is available on the changes that occur in gene expression during low-calorie diets in humans. Of the 38 genes that were selected based on their importance in adipose tissue biology (Table 1), none were differentially regulated according to the fat and carbohydrate content of the diet. Adjustment for baseline covariates did not alter the outcome of this analysis. However, ten genes were shown to be regulated by calorie restriction. Therefore, the main finding of this extensive mRNA study is the lack of effect of macronutrient composition and the predominant impact of energy restriction and/or weight loss on adipose tissue gene expression. The molecular pathways that mediate the effects of fatty acids and glucose on gene transcription have been partially characterised [16–18]. Several of the genes investigated in this study are directly regulated by fatty acids (adipocyte lipid binding protein, uncoupling protein 2 and angiotensinogen) and glucose (leptin, plasminogen activator inhibitor 1, fatty acid synthase and hormone-sensitive lipase) in adipocytes [25–31]. The greater reduction in plasma NEFA levels in the low-fat group compared with the high-fat group had no impact on gene expression. The changes in gene expression may therefore be related to energy restriction and/or a decrease in fat mass. These two parameters may regulate mRNA levels via different mechanisms. In the short term, fasting induces the expression of hormone-sensitive lipase and uncoupling protein 2 mRNAs [32, 33], whereas moderately hypocaloric diets of longer duration decrease the levels of these two transcripts (Table 3). It may be hypothesised that an acute negative energy balance upregulates the expression of the gene encoding uncoupling protein 2 through an increase in plasma NEFA levels, whereas a reduction in fat mass has a negative effect on the expression of this gene [34]. Recent work has shown that regulators of lifespan and mediators of the effect of calorie restriction may be common to yeast, Caenorhabditis elegans and mammals [35]. These regulators, including sirtuin 1 (the mammalian orthologue of the yeast NAD-dependent deacetylase SIR2) and the FOXO transcription factors of the forkhead family which mediate some of the transcriptional effects of insulin, may play an important role in altering metabolism in response to calorie restriction. It has recently been shown that sirtuin 1 is activated during calorie restriction in adipose tissue and attenuates adipogenesis through repression of PPARγ [20]. Our data raise the possibility that the sirtuin 1 and/or FOXO pathways are involved in the regulation of adipose tissue gene expression during hypocaloric diets in humans. However, the direct involvement of these pathways remains to be demonstrated.
The ten genes that were shown to be regulated by energy restriction belong to different functional categories. Several genes encoding secreted proteins were regulated. Leptin levels have previously been shown to decrease during a low-calorie diet [36]. We previously observed a 33% decrease in adipose tissue leptin mRNA levels during a very-low-calorie diet which was accompanied by a 29% decrease in circulating leptin levels [10]. Adiponectin is an abundant adipose-tissue-secreted protein that plays important roles in glucose and lipid metabolism in both skeletal muscle and liver. It has been suggested that obesity and insulin resistance are associated with decreased adiponectin levels [37]. However, the increased insulin sensitivity observed during calorie restriction is not associated with changes in plasma levels of adiponectin or the expression of the adiponectin gene in adipose tissue [14]. Accordingly, levels of adiponectin mRNA were not affected by the hypocaloric diets in this study, whereas insulin sensitivity was improved. The mRNA levels of the two recently characterised adiponectin receptors (adiponectin receptors 1 and 2) were not regulated by either of the two diets [38]. Hierarchical clustering suggests a role for liver X receptor α in the regulation of the genes encoding the two adiponectin receptors, as these three genes showed highly similar patterns of expression during the diets (Fig. 1). The expression of liver X receptor α is induced during adipocyte differentiation, and this transcription factor seems to regulate a subset of genes involved in lipid metabolism [39]. It may therefore be of interest to determine whether the adiponectin receptors are direct targets of liver X receptor α, as has previously been shown by a DNA-array-based global gene expression profiling experiment using macrophages [40]. Osteonectin, which is also known as secreted protein, acidic and rich in cysteine (SPARC), is involved in extracellular matrix remodelling, and osteonectin-deficient mice exhibit increased fat deposition [41]. Furthermore, levels of adipose tissue osteonectin mRNA have been observed to be increased in ob/ob mice and in animals with diet- or drug-induced obesity relative to those in lean mice [42]. Here, we show that weight loss is accompanied by a downregulation of osteonectin gene expression.
The negative energy balance produced by a hypocaloric diet is known to profoundly modify adipocyte metabolism, particularly the lipolytic pathway [43]. Insulin counteracts lipolysis, mainly through activation of phosphodiesterase 3B [44]. The slight decrease in phosphodiesterase 3B mRNA levels observed in the present study could favour an increase in fat cell cAMP levels and thus enhance lipolysis. However, the decreased levels of hormone-sensitive lipase and natriuretic peptide type A receptor (which mediates cyclic GMP-dependent activation of lipolysis in human adipocytes [45]) mRNAs indicate a decreased lipolytic capacity. In addition, the expression of hormone-sensitive lipase is reportedly decreased following weight loss programmes [46, 47]. The downregulation of a key enzyme responsible for triglyceride breakdown may counterbalance the decrease in lipoprotein lipase mRNA levels. Lipoprotein lipase controls the entry of fatty acids derived from chylomicrons and VLDL into the fat cell. In morbidly obese subjects, weight loss leads to decreases in lipoprotein lipase mRNA levels and the activity of this enzyme [48]. Fatty acid uptake from extracellular sources is partially mediated by transmembrane transporters of fatty acids. The key proteins involved are plasma membrane fatty acid binding protein, fatty acid translocase/CD36 and fatty acid transport protein 1. These transporters may be regulated by nutritional challenges [49]. In the present study, fatty acid translocase/CD36 mRNA levels were significantly decreased, whereas the expression of the other transporters and the intracellular adipocyte fatty acid binding protein gene was not affected. As shown in Fig. 1, PPARα is coexpressed with the genes encoding the fatty acid transporters, plasma membrane fatty acid binding protein and fatty acid transport protein 1. This suggests that, in human adipose tissue, PPARα regulates the transcription of the fatty acid transporter genes. In support of the data, the fatty acid transport protein 1 promoter contains a functional PPARα response element [50].
Several classes of transcription factors and nuclear factors have been implicated in the control of adipogenesis. One of the four master nodes in the cluster analysis (Fig. 1) contained the transcripts for the transcription factors PPARγ, PPARβ and sterol regulatory element binding protein 1c, all of which have been shown to play critical roles in adipocyte differentiation and lipogenesis [51, 52]. Two protein isoforms of PPARγ that differ in their amino terminus region have been characterised: PPARγ1 is expressed in several cell types, including adipocytes, whereas PPARγ2 is almost exclusively expressed in adipose tissue. The relative changes in the levels of expression of PPARγ (PPARγ plus PPARγ2) and PPARγ2 were clustered together in a node containing the fatty acid translocase/CD36 (Fig. 1), which is a well known target for PPARγ in adipose tissue [53, 54]. The decreased PPARγ mRNA levels observed in the present study are consistent with previous reports [10, 55]. As fatty acid translocase/CD36 and lipoprotein lipase are direct targets of PPARγ [54, 56], their downregulation may be related to the decreased expression of this transcription factor.
The expression of the gene encoding PGC-1α, a co-activator of PPARγ, was upregulated. This was the only gene induced by the diets studied. Together with several classes of transcription factor, PGC-1α controls the expression of genes involved in mitochondrial oxidative phosphorylation [57], and it has been shown to promote mitochondriogenesis in several tissues. The expression of PGC-1α in human white adipocytes induces the expression of respiratory chain proteins, fatty acid oxidation enzymes and brown adipocyte markers [58]. The upregulation of this coactivator after a relatively short period of calorie restriction may be viewed as an early adaptation of the transcriptional activation of genes involved in mitochondrial energy metabolism that occurs after long-term calorie restriction [59].
In summary, our data show the high-fat and low-fat low-calorie diets produce similar reduction in anthropometric parameters, including fat mass. In line with this, we observed no differences in adipose tissue gene expression between the two diets. Moderate energy restriction induced an increase in the expression of PGC-1α, which encodes a transcriptional coactivator that is linked to energy metabolism, and a decrease in the expression of genes involved in lipid metabolism. The present study provides evidence that energy restriction rather than the fat: carbohydrate ratio of a low-calorie diet is of importance in the regulation of transcription in the human adipocyte.
Abbreviations
- NUGENOB:
-
Nutrient–Gene Interactions in Human Obesity—Implications for Dietary Guidelines
- PGC-1α:
-
peroxisome proliferator-activated receptor γ co-activator 1α
- PPAR:
-
peroxisome proliferator-activated receptor
References
Gelfand RA, Hendler R (1989) Effect of nutrient composition on the metabolic response to very low calorie diets: learning more and more about less and less. Diabetes/Metab Rev 5:17–30
Shah M, Garg A (1996) High-fat and high-carbohydrate diets and energy balance. Diabetes Care 19:1142–1152
Lewis SB, Wallin JD, Kane JP, Gerich JE (1977) Effect of diet composition on metabolic adaptations to hypocaloric nutrition: comparison of high carbohydrate and high fat isocaloric diets. Am J Clin Nutr 30:160–170
Alford BB, Blankenship AC, Hagen RD (1990) The effects of variations in carbohydrate, protein, and fat content of the diet upon weight loss, blood values, and nutrient intake of adult obese women. J Am Diet Assoc 90:534–540
Golay A, Eigenheer C, Morel Y, Kujawski P, Lehmann T, de Tonnac N (1996) Weight-loss with low or high carbohydrate diet? Int J Obes Relat Metab Disord 20:1067–1072
Lean ME, Han TS, Prvan T, Richmond PR, Avenell A (1997) Weight loss with high and low carbohydrate 1200 kcal diets in free living women. Eur J Clin Nutr 51:243–248
McManus K, Antinoro L, Sacks F (2001) A randomized controlled trial of a moderate-fat, low-energy diet compared with a low-fat, low-energy diet for weight loss in overweight adults. Int J Obes Relat Metab Disord 25:1503–1511
Vidal-Puig AJ, Considine RV, Jimenez-Liñan M et al (1997) Peroxisome proliferator-activated receptor gene expression in human tissues. Effects of obesity, weight loss, and regulation by insulin and glucocorticoids. J Clin Invest 99:2416–2422
Barbe P, Millet L, Larrouy D et al (1998) Uncoupling protein-2 messenger ribonucleic acid expression during very-low-calorie diet in obese premenopausal women. J Clin Endocrinol Metab 83:2450–2453
Bastard JP, Hainque B, Dusserre E et al (1999) Peroxisome proliferator activated receptor-gamma, leptin and tumor necrosis factor-alpha mRNA expression during very low calorie diet in subcutaneous adipose tissue in obese women. Diabetes/Metab Res Rev 15:92–98
Bastard JP, Jardel C, Bruckert E et al (2000) Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab 85:3338–3342
Bastard JP, Vidal H, Jardel C et al (2000) Subcutaneous adipose tissue expression of plasminogen activator inhibitor-1 gene during very low calorie diet in obese subjects. Int J Obes Relat Metab Disord 24:70–74
Richelsen B, Pedersen SB, Kristensen K et al (2000) Regulation of lipoprotein lipase and hormone-sensitive lipase activity and gene expression in adipose and muscle tissue by growth hormone treatment during weight loss in obese patients. Metabolism 49:906–911
Garaulet M, Viguerie N, Porubsky S et al (2004) Adiponectin gene expression and plasma values in obese women during very-low-calorie diet. Relationship with cardiovascular risk factors and insulin resistance. J Clin Endocrinol Metab 89:756–760
Stich V, Marion-Latard F, Hejnova J et al (2002) Hypocaloric diet reduces exercise-induced alpha 2-adrenergic antilipolytic effect and alpha 2-adrenergic receptor mRNA levels in adipose tissue of obese women. J Clin Endocrinol Metab 87:1274–1281
Duplus E, Glorian M, Forest C (2000) Fatty acid regulation of gene transcription. J Biol Chem 275:30749–30752
Pégorier J, May C, Girard J (2004) Control of gene expression by fatty acids. J Nutr 134:S2444–S2449
Yamashita H, Takenoshita M, Sakurai M et al (2001) A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc Natl Acad Sci USA 98:9116–9121
Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K (2004) Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc Natl Acad Sci USA 101:7281–7286
Picard F, Kurtev M, Chung N et al (2004) Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 429:771–776
Cohen HY, Miller C, Bitterman KJ et al (2004) Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305:390–392
Vickers AJ, Altman DG (2001) Statistics notes: analysing controlled trials with baseline and follow up measurements. BMJ 323:1123–1124
Ward JH (1963) Hierarchical grouping to optimize an objective function. J Am Stat Assoc 58:236–244
Gordon AD (1999) Hierarchical classifications. In: Classification, 2nd revised edn. CRC press, London, pp 69–111
Amri E-Z, Bertrand B, Ailhaud G, Grimaldi P (1991) Regulation of adipose cell differentiation. I. Fatty acids are inducers of the aP2 gene expression. J Lipid Res 32:1449–1456
Safonova I, Aubert J, Negrel R, Ailhaud G (1997) Regulation by fatty acids of angiotensinogen gene expression in preadipose cells. Biochem J 322:235–239
Viguerie-Bascands N, Saulnier-Blache JS, Dandine M, Dauzats M, Daviaud D, Langin D (1999) Increase in uncoupling protein-2 mRNA expression by BRL49653 and bromopalmitate in human adipocytes. Biochem Biophys Res Commun 256:138–141
Gabriely I, Yang XM, Cases JA, Ma XH, Rossetti L, Barzilai N (2002) Hyperglycemia induces PAI-1 gene expression in adipose tissue by activation of the hexosamine biosynthetic pathway. Atherosclerosis 160:115–122
Smih F, Rouet P, Lucas S et al (2002) Transcriptional regulation of adipocyte hormone-sensitive lipase by glucose. Diabetes 51:293–300
Zhang P, Klenk ES, Lazzaro MA, Williams LB, Considine RV (2002) Hexosamines regulate leptin production in 3T3-L1 adipocytes through transcriptional mechanisms. Endocrinology 143:99–106
Rumberger JM, Wu T, Hering MA, Marshall S (2003) Role of hexosamine biosynthesis in glucose-mediated up-regulation of lipogenic enzyme mRNA levels: effects of glucose, glutamine, and glucosamine on glycerophosphate dehydrogenase, fatty acid synthase, and acetyl-CoA carboxylase mRNA levels. J Biol Chem 278:28547–28552
Millet L, Vidal H, Andreelli F et al (1997) Increased uncoupling protein-2 and -3 mRNA expression during fasting in obese and lean humans. J Clin Invest 100:2665–2670
Vidal H, Langin D, Andreelli F, Millet L, Larrouy D, Laville M (1999) Lack of skeletal muscle uncoupling protein 2 and 3 mRNA induction during fasting in type-2 diabetic subjects. Am J Physiol 277:E830–E837
Langin D (2003) The role of uncoupling protein 2 in the development of type 2 diabetes. Drugs Today 39:287–295
Koubova J, Guarente L (2003) How does calorie restriction work? Genes Dev 17:313–321
Wadden TA, Considine RV, Foster GD, Anderson DA, Sarwer DB, Caro JS (1998) Short- and long-term changes in serum leptin dieting obese women: effects of caloric restriction and weight loss. J Clin Endocrinol Metab 83:214–218
Matsuzawa Y, Funahashi T, Kihara S, Shimomura I (2004) Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol 24:29–33
Yamauchi T, Kamon J, Ito Y et al (2003) Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423:762–769
Hummasti S, Laffitte BA, Watson MA et al (2004) Liver X receptors are regulators of adipocyte gene expression but not differentiation: identification of apoD as a direct target. J Lipid Res 45:616–625
Chinetti G, Zawadski C, Fruchart JC, Staels B (2004) Expression of adiponectin receptors in human macrophages and regulation by agonists of the nuclear receptors PPARalpha, PPARgamma, and LXR. Biochem Biophys Res Commun 314:151–158
Bradshaw AD, Graves DC, Motamed K, Sage EH (2003) SPARC-null mice exhibit increased adiposity without significant differences in overall body weight. Proc Natl Acad Sci USA 100:6045–6050
Tartare-Deckert S, Chavey C, Monthouel M-N, Gautier N, Van Obberghen E (2001) The matricellular protein SPARC/osteonectin as a newly identified factor up-regulated in obesity. J Biol Chem 276:22231–22237
Langin D, Lucas S, Lafontan M (2000) Millenium fat cell lipolysis reveals unsuspected novel tracks. Horm Metab Res 32:443–452
Eriksson H, Ridderstråle M, Degerman E et al (1995) Evidence for the key role of the adipocyte cGMP-inhibited cAMP phosphodiesterase in the antilipolytic action of insulin. Biochim Biophys Acta 1266:101–107
Sengenes C, Bouloumie A, Hauner H et al (2003) Involvement of a cGMP-dependent pathway in the natriuretic peptide-mediated hormone-sensitive lipase phosphorylation in human adipocytes. J Biol Chem 278:48617–48626
Reynisdottir S, Langin D, Carlström K, Holm C, Rössner S, Arner P (1995) Effects of weight reduction on the regulation of lipolysis in adipocytes of women with upper-body obesity. Clin Sci 89:421–429
Kolehmainen M, Vidal H, Ohisalo JJ, Pirinen E, Alhava E, Uusitupa MIJ (2002) Hormone sensitive lipase expression and adipose tissue metabolism show gender difference in obese subjects after weight loss. Int J Obes Relat Metab Disord 26:6–16
Kern PA, Ong JM, Saffari B, Carty J (1990) The effects of weight loss on the activity and expression of adipose-tissue lipoprotein lipase in very obese humans. N Engl J Med 322:1053–1059
Greenwalt DE, Scheck SH, Rhinehart-Jones T (1995) Heart CD36 expression is increased in murine models of diabetes and in mice fed a high fat diet. J Clin Invest 96:1382–1388
Frohnert BI, Hui TY, Bernlohr DA (1999) Identification of a functional peroxisome proliferator-responsive element in the murine fatty acid transport protein gene. J Biol Chem 274:3970–3977
Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM (2000) Transcriptional regulation of adipogenesis. Genes Dev 14:1293–1307
Holst D, Grimaldi PA (2002) New factors in the regulation of adipose differentiation and metabolism. Curr Opin Lipidol 13:241–245
Motojima K, Passilly P, Peters JM, Gonzalez FJ, Latruffe N (1998) Expression of putative fatty acid transporter genes are regulated by peroxisome proliferator-activated receptor alpha and gamma activators in a tissue- and inducer-specific manner. J Biol Chem 273:16710–16714
Sato O, Kuriki C, Fukui Y, Motojima K (2002) Dual promoter structure of mouse and human fatty acid translocase/CD36 genes and unique transcriptional activation by peroxisome proliferator-activated receptor alpha and gamma ligands. J Biol Chem 277:15703–15711
Vidal-Puig A, Jimenez-Linan M, Lowell BB et al (1996) Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J Clin Invest 97:2553–2561
Schoonjans K, Peinado-Onsurbe J, Lefebvre A-M et al (1996) PPARα and PPARγ activators direct a distinct tissue specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J 15:5336–5348
Puigserver P, Spiegelman BM (2003) Peroxisome proliferator-activated receptor-gamma coactivator 1α (PGC-1α): transcriptional coactivator and metabolic regulator. Endocr Rev 24:78–90
Tiraby C, Tavernier G, Lefort C et al (2003) Acquirement of brown fat cell features by human white adipocytes. J Biol Chem 278:33370–33376
Higami Y, Pugh TD, Page GP, Allison DB, Prolla TA, Weindruch R (2004) Adipose tissue energy metabolism: altered gene expression profile of mice subjected to long-term caloric restriction. FASEB J 18:415–417
Acknowledgements
We would like to thank S. Dejean (Department of Statistics, UMR C5583 UPS–CNRS–INSA, Paul Sabatier University, Toulouse) for clustering analyses. The NUGENOB study is supported by the European Community (Contract no. QLK1-CT-2000-00618). The authors have declared that there are no conflicts of interest regarding this work.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Viguerie, N., Vidal, H., Arner, P. et al. Adipose tissue gene expression in obese subjects during low-fat and high-fat hypocaloric diets. Diabetologia 48, 123–131 (2005). https://doi.org/10.1007/s00125-004-1618-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-004-1618-x